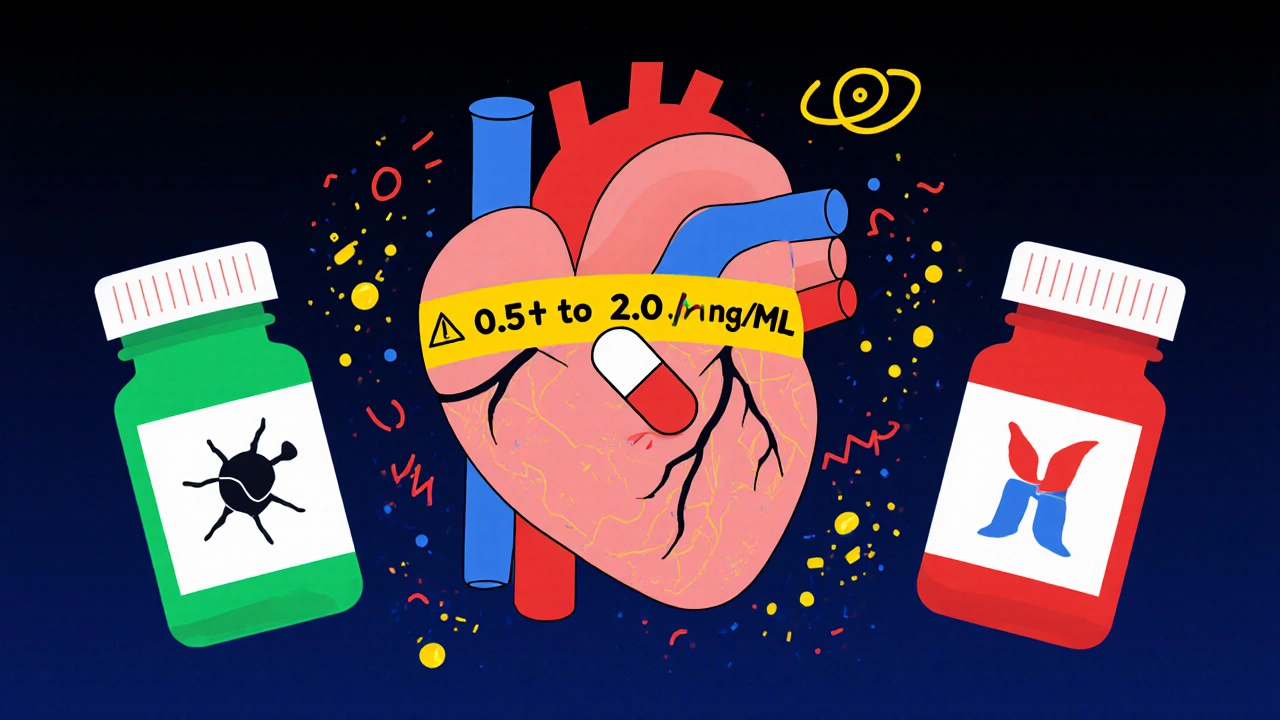
When you take digoxin, even a tiny change in how much of the drug enters your bloodstream can mean the difference between healing and hospitalization. This isn’t just any medication-it’s a digoxin generic, one of the most dangerous drugs in the narrow therapeutic index (NTI) club. The gap between too little and too much is razor-thin: a blood level below 0.5 ng/mL might not help your heart, but above 2.0 ng/mL? That’s when nausea, confusion, irregular heartbeat, and even death can creep in. And here’s the real problem: not all generic digoxin tablets behave the same way in your body, even if they’re labeled the same.
Why Digoxin Is Different from Other Generics
Most generic drugs are safe to swap. You can switch from one brand of ibuprofen to another and barely notice a difference. But digoxin? It’s not like that. Because it has such a narrow window between effective and toxic, the FDA treats it like a high-risk drug-even more strictly than most NTI medications like warfarin or lithium. In 2002, the agency made a rare move: it required every generic digoxin product to prove it delivers the same amount of drug into the bloodstream as the brand-name version, Lanoxin. That’s not standard for most generics. Most only need to match within 80-125% of the original. For digoxin, that’s the rule. But here’s the catch: that rule applies to one generic compared to Lanoxin, not to one generic compared to another generic.What Bioavailability Really Means for You
Bioavailability is just a fancy word for how much of the drug actually gets into your blood after you swallow it. For digoxin tablets, the average is about 60-80%. But that number can swing wildly from person to person. One study showed that in healthy volunteers, some people absorbed only 45% of the dose, while others absorbed over 90%. The average might still meet FDA standards, but if you’re the person who only absorbs half, you’re getting half the benefit-and if you switch to a different generic that’s absorbed better, you could suddenly overdose. Even the form matters. Digoxin elixir (the liquid version) is absorbed better than tablets-up to 70-85% of the dose. So if you switch from a tablet to a liquid, or vice versa, your blood levels can shift without any dose change. That’s why doctors don’t just look at the pill you’re holding-they look at how your body reacts to it.The Hidden Risk: Switching Between Generics
Most patients never think twice about switching from one pharmacy’s generic to another. Pharmacists often do it automatically to save money. But for digoxin, that’s a gamble. There are no studies proving that Generic A is the same as Generic B. The FDA only tested each generic against Lanoxin, not against each other. So if you’ve been stable on Generic A for six months, and your insurance switches you to Generic B without telling you, your digoxin level could drop 20% or spike 30%. That’s enough to cause heart failure to worsen-or trigger a dangerous arrhythmia. Real-world cases back this up. There are published reports of elderly patients admitted to the hospital after switching generics. One man had been stable for years on a generic digoxin tablet. His pharmacy switched him to a different brand. Three days later, he was vomiting, seeing yellow halos around lights (a classic sign of digoxin toxicity), and his heart rate was erratic. His blood level? 3.1 ng/mL-well above toxic. He had no other changes in meds or kidney function. Just a different pill.
Who’s Most at Risk?
Older adults make up the majority of digoxin users. They’re more likely to have reduced kidney function, which slows how fast the drug leaves the body. They’re also more likely to be on multiple medications that interact with digoxin-like antibiotics, diuretics, or heart rhythm drugs. Even small changes in kidney function or new drug interactions can push digoxin levels into danger zones. And because older patients often take their meds without supervision, they might not notice early symptoms like fatigue, dizziness, or loss of appetite until it’s too late. Patients with atrial fibrillation or heart failure are especially vulnerable. Digoxin doesn’t cure these conditions-it helps manage them. If the drug level dips too low, their heart rate can race uncontrollably. If it climbs too high, their heart can start skipping beats in dangerous patterns. Neither outcome is acceptable.How to Stay Safe: Monitoring Is Non-Negotiable
The only reliable way to know if your digoxin dose is right is to check your blood level. And not just once. The American College of Clinical Pharmacy says you need a trough level-drawn just before your next dose-after you start the drug, after any dose change, and after switching manufacturers. The ideal range? 0.5-2.0 ng/mL. But newer research suggests that for heart failure patients, staying between 0.5 and 0.9 ng/mL lowers the risk of death. That’s why your doctor might aim lower than you expect. If you’ve switched generics, get your level checked 3-5 days later. That’s how long it takes for digoxin to reach steady state in your body. Don’t wait for symptoms. By then, it might be too late. Also, get checked whenever your kidney function changes-like after a bout of dehydration, infection, or starting a new diuretic. Even a small drop in kidney filter rate can cause digoxin to build up.
What You Can Do Right Now
If you’re on digoxin, here’s what to do:- Know which generic you’re on. Write down the manufacturer name on the bottle. If it changes, ask why.
- Never switch between generics without telling your doctor. Even if your pharmacist says it’s the same.
- Keep a log of symptoms: nausea, vision changes, irregular heartbeat, dizziness, fatigue.
- Ask for a digoxin blood test after any switch, even if you feel fine.
- If you’re on the liquid form, never switch to tablets without a dose review. The absorption is different.
The Bottom Line: Consistency Saves Lives
Yes, generic digoxin is cheaper. And yes, most generics meet FDA standards. But the system was built for drugs that can tolerate variation. Digoxin can’t. The FDA knows this. That’s why it’s one of the few drugs with an “AB” rating in the Orange Book-meaning it’s approved as bioequivalent to the brand. But that’s only half the story. The real risk isn’t between brand and generic. It’s between one generic and another. The best advice from the American Heart Association and the American College of Cardiology is simple: use the same manufacturer’s product every time. If you can’t, monitor closely. Don’t assume safety because it’s labeled “generic.” Don’t trust the pharmacy’s substitution. Your life depends on consistency.There’s no magic pill here. No shortcut. Just careful attention, regular blood tests, and a willingness to speak up when something changes. That’s how you stay safe with digoxin.
Are all generic digoxin tablets the same?
No. While each generic must prove it’s bioequivalent to the brand-name Lanoxin, there are no studies comparing one generic to another. Two different generics may both be approved, but they can still behave differently in your body. Switching between them can cause dangerous changes in blood levels.
How often should digoxin blood levels be checked?
Check levels when you first start digoxin, after any dose change, and always after switching manufacturers or formulations (tablet to liquid, for example). Also check after any change in kidney function, new medications, or if you develop symptoms like nausea, dizziness, or irregular heartbeat. A trough level (drawn just before your next dose) is standard.
Can I switch from a brand-name to a generic without risk?
It’s possible, but not risk-free. Even though generics are required to meet FDA bioequivalence standards, individual responses vary. Always check your blood level 3-5 days after switching. Your doctor may adjust your dose based on the result, even if the label says it’s the same strength.
Why is digoxin elixir more bioavailable than tablets?
The liquid form dissolves more completely and quickly in the stomach, leading to better and more consistent absorption. Tablets rely on how well they break down in the gut, which can be affected by food, stomach acid, or age-related changes. Elixir typically delivers 70-85% of the dose, while tablets average 60-80%.
What should I do if my pharmacy switches my digoxin without telling me?
Call your doctor immediately. Don’t wait for symptoms. Ask for a digoxin blood level test within 3-5 days. Bring the new bottle to your appointment so your doctor knows exactly what you’re taking. You have the right to request the same manufacturer if you’re stable on it.
Is digoxin still used today, or is it outdated?
Digoxin is still used, especially for heart failure with atrial fibrillation, when other drugs aren’t enough. It’s not a first-line treatment anymore, but it remains valuable for symptom control in specific patients. Its use has declined due to safety concerns, but it’s far from obsolete-especially in older adults with limited treatment options.


Scott McKenzie
Just got my digoxin switched last week without a heads-up. Went from Teva to Mylan. Felt fine at first, but then my heart started acting up like it was trying to tap dance. Got my levels checked - 2.8 ng/mL. Scared the hell out of me. Now I write the manufacturer on my pill bottle with a Sharpie. 🚨💊